working
with tumoroids
in the clinostar
What is a cancerous tumoroid?
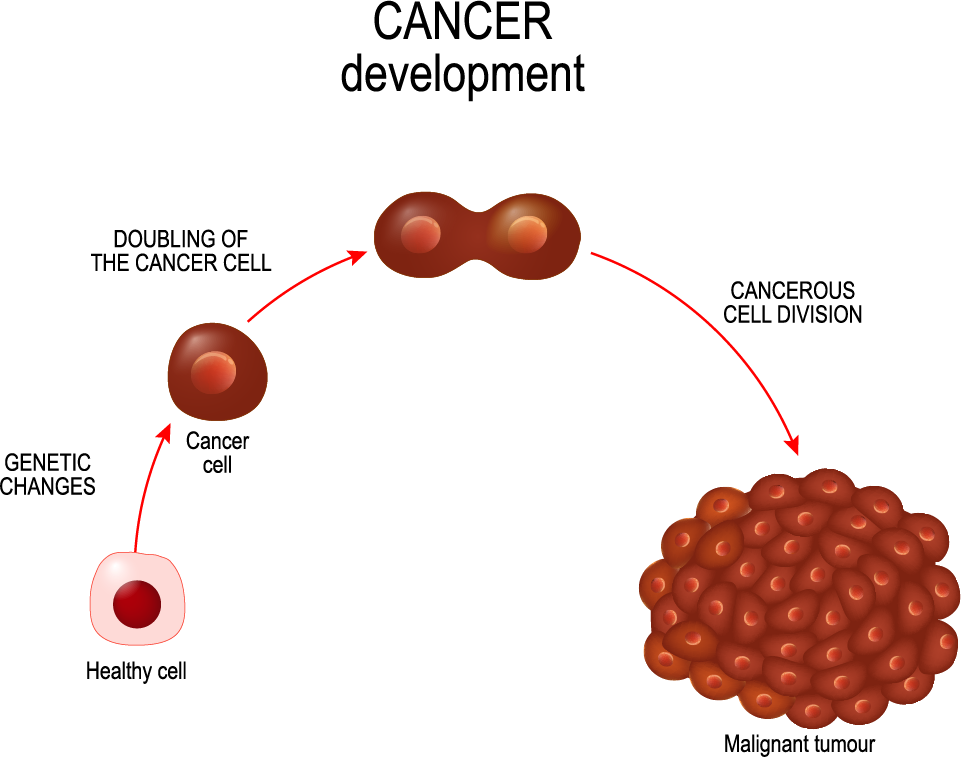
As cancer cells accumulate changes in their genetic makeup (mutations) and how genes are regulated (epigenetic alterations, red), they gradually relinquish the intricate controls that usually manage their behavior. This loss of control sets the stage for cells to multiply uncontrollably and adopt invasive characteristics, ultimately coalescing into tumoroids. These tumoroids are a significant menace to the body’s overall balance.
In the evolving landscape of oncology, the concept of “cancerous tumoroids” is gaining recognition, denoting irregular growths distinguished by their unregulated cell division and tendency to invade surrounding tissues. These growths prominently display the key indicators of malignancy, showcasing the capability to spread to distant sites through metastasis, thereby perturbing the usual arrangement of healthy tissues.
White Paper
Start your journey of designing relevant in vitro models for optimal cancer research.
Read about cell culture techniques and how to validate cell culture models.

How Christophe Deben cultivates tumoroids in the ClinoStar
See how Christophe Deben from the Center of Oncological Research at Antwerpen University uses the ClinoStar to cultivate organoids. Get insights into how Christophe and his team aim to introduce patient-derived organoids in preclinical and clinical developments.
How to work with cancer cells in 3D cell culture
Two methods are commonly employed when creating cancer spheroids: Scaffold-Based Systems and Spheroids.
- Scaffold-Based Systems: This technique utilizes scaffold materials like hydrogels, porous polymers, or natural extracellular matrix components (such as collagen or Matrigel). These scaffolds serve as supportive frameworks. Initially, cancer cells are combined with the scaffold material and then placed within a suitable culture vessel. The scaffold is a platform for cells to anchor onto and grow three-dimensional. Over time, cells proliferate into a 3D structure resembling the growth pattern of tumors in the body.
- Scaffold-Free Systems: Organoids or spheroids are formed when cancer cells naturally aggregate into spherical clusters. This is achieved by culturing cells in suspension, meaning they are not attached to a surface.
Both approaches offer insights into cancer cell behavior in a more realistic 3D environment, enhancing our understanding of their growth and response to treatment. Scaffold-based systems provide a structured foundation for cell growth, while Scaffold-free systems involve cells uniting naturally in a suspended environment, akin to forming clusters resembling small tumors. These methods, including the innovative use of rotary bioreactors, contribute to our knowledge of cancer biology and therapeutic strategies.
How to work with cancer organoids in the ClinoStar
Utilizing the Clinostar for cancer tumoroid production enhances consistency, gas, and nutrient exchange in simulated microgravity conditions. This, in turn, allows for very long-term cultures (up to a year or more), providing a more accurate and informative platform for studying cancer cell behavior. The unique advantages of the Clinostar contribute not only to terrestrial cancer research but also offer insights into how cancer cells might respond in space-related contexts.
Using a rotary bioreactor, like the ClinoStar, gives scientists distinct advantages when producing cancer tumoroids. An example is that the controlled rotation of the ClinoStar promotes uniform cell aggregation, resulting in consistent tumoroid size and structure. The long-term culture allows the growth rate of the cancer cells to fall to that seen in cancers in vivo. These features ensure that experimental results are reliable, reproducible, and relevant.

NEWSLETTER
Keep in touch
Make sure you never miss a message.
Sign up for CelVivo’s monthly newsletter and get all the latest ClinoStar research, 3D cell culture trends, insightful symposiums, and exclusive webinars.
ClinoStar Research
Get inspired by the newest research made with the ClinoStar system.
A Novel NCI-H69AR Drug-Resistant Small-Cell Lung Cancer Mini-Tumor Model for Anti-Cancer Treatment Screening
DOI: 10.3390/cells12151980
Small-cell lung cancer is a fast-growing carcinoma with a poor prognosis and a high level of relapse due to multi-drug resistance (MDR).

Investigation of reversible histone acetylation and dynamics in gene expression regulation using 3D liver spheroid model
DOI: 10.1186/s13072-022-00470-7
A demonstration, that 3D liver spheroids are a suitable system to model chromatin dynamics and response to epigenetics inhibitors.
A novel NCI-H69V small cell lung cancer functional mini-tumor model for future treatment screening applications
DOI: 10.1002/btpr.3253
In vitro cancer models are crucial in chemotherapy development, and three-dimensional (3D) models aim to bridge the gap between two-dimensional (2D) flat cultures and in vivo testing.
Start optimising your
In Vitro models today