Growing
brain organoids
in the clinostar
What are brain organoids?
Brain organoids are a 3D structure typically grown in vitro from pluripotent stem cells and are engineered to resemble certain aspects of the developing human brain.
By exposing stem cells to specific growth factors and other signaling molecules in a temporal sequence and a controlled environment, researchers can coax them to differentiate into brain cells and self-organize into brain-like structures.
These neural organoids are small, approximately pea-sized, and contain different types of brain cells, including neurons, astrocytes, and oligodendrocytes. Given the paucity of available human brain material, organoids can be used as models to study development, test drug efficacy and toxicity, and investigate the underlying mechanisms of neurodegenerative diseases and neurological disorders. While these organoids are not exact replicas of the human brain, they can provide a readily available tool in a more controlled and ethically acceptable manner than animal models or human embryos.
Transitioning from 2D to 3D
Learn how Marina from University of Groningen has created brain organoids with the ClinoStar, resulting in a smaller necrotic core compared to petri dishes.
Growing neural organoids in 3D
Organoids can be expanded and maintained in 3D culture systems. As a result, the expansion allows for the formation of cell-cell and cell-matrix interactions. This interaction is crucial for the formation of complex tissue structures.
One method for growing neural organoids in 3D culture is hydrogels. Hydrogels are three-dimensional networks of hydrophilic polymers that can mimic the extracellular matrix (ECM) found in vivo. Another approach is to use microfluidic devices, which can provide precise control over the chemical and physical environment of the organoid.
Clinostat 3D culture systems allow researchers to generate large and complex organoids to better model the human brain. However, the complexity and heterogeneity of brain organoids can present challenges due to standardization, reproducibility, and the interpretation of experimental results.
White Paper
Start your journey of growing neural organoids in the ClinoStar system today.
Read how other researchers are growing organoids in 3D and learn about their protocols.

How to grow brain organoids
in the ClinoStar
The ClinoStar is particularly well-suited for growing and expanding brain organoids. It provides a controlled and physiologically relevant culture environment for these complex structures, which can be maintained for weeks or months.
It can also be used to scale up the production of neural organoids for drug discovery and other applications. With the ClinoStar system, you can generate large numbers of organoids consistent in size and structure. These organoids can improve the reproducibility of experiments and facilitate higher-throughput drug screening.

Transforming blood cells to human brain organoids
Meet a:head Bio. An Austrian company working with CNS therapeutics, transforming human blood cells into brain organoids and the European winners of a ClinoStar 2 trial in 2024. Hear from CEO, Oliver Szolar, how a:head will be using the ClinoStar 2 in their research.
ClinoStar Research
Get inspired by the newest research made with the ClinoStar system.
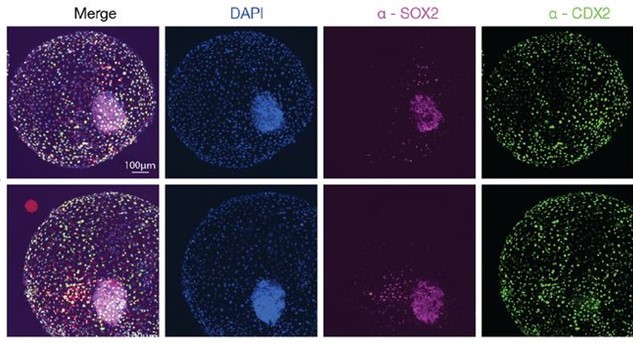
Bovine blastocyst like structures derived from stem cell cultures
DOI: https://doi.org/10.1101/2022.12.20.521301
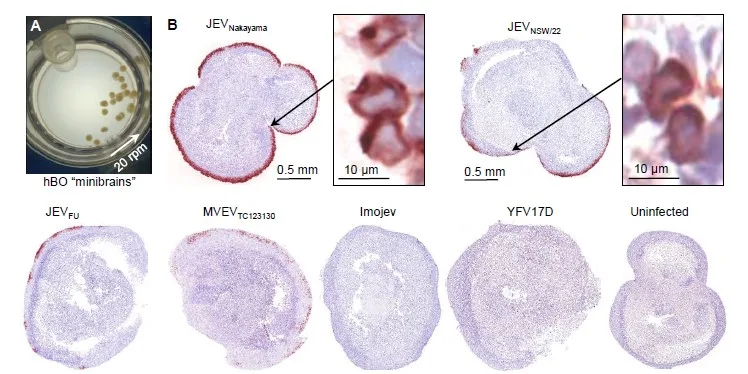
Characterization of an Australian outbreak Japanese Encephalitis virus genotype 4 isolate in mice and human cortical brain organoids
DOI: 10.1101/2023.04.26.538504
Researchers characterized the neuropathology of JEV in adult mice and found that the virus caused neuronal degeneration, leukocyte infiltrates, and other brain lesions. The virus was also lethal to some mice and destroyed human neuronal cells in vitro.
Omicron BA.5 infects human brain organoids and is neuroinvasive and lethal in K18-hACE2 mice
DOI: 10.1101/2022.12.22.521696
NEWSLETTER
Keep in touch
Make sure you never miss a message.
Sign up for CelVivo’s monthly newsletter and get all the latest ClinoStar research, 3D cell culture trends, insightful symposiums, and exclusive webinars.
Start optimising your
In Vitro models today