Blog: Refining Biomedical Research Beyond Animal Models
What the Pivot to Human-Based Technologies Means for Science and How 3D Cellular Constructs like Organoids Are Part of the Solution
By Stephen J. Fey, PhD, Chief Research Officer, CelVivo
April of 2025 can fittingly be described as a turning point for how we will create the treatments of the future, with two landmark decisions that ought to be centre stage for everyone in the industry. First, the U.S. Food and Drug Administration (FDA) announced plans to phase out the requirement for animal testing of certain classes of new drugs – notably monoclonal antibody (mAb) therapies – in favour of “more effective, human-relevant methods.” Then, on April 29th, the National Institutes of Health (NIH) announced a new initiative to prioritize human-based technologies and reduce animal use in testing along with the aim of establishing the Office of Research Innovation, Validation, and Application (ORIVA) to scale-up the use of non-animal approaches. Both of these initiatives reflect a growing regulatory push to replace traditional animal models with advanced in vitro systems, but realizing the underlying vision comes with practical challenges that require new solutions.
In this article, we explain how clinostat-based three-dimensional (3D) culture of different cellular constructs can refine biomedical research and reduce reliance on animal models.
What Is Driving the Decisions
The new initiatives follow the track of the FDA Modernization Act 2.0 signed in late 2022, aiming to improve the predictive accuracy of preclinical research while addressing ethical and cost concerns associated with animal testing. Organoid, spheroid and other cellular clusters (e.g.: bio-printed constructs) that mimic aspects of human tissue architecture and function (hereafter ‘organoids’)– have emerged as promising human-relevant models to meet this ambition.
However, a challenge lies in organoids being inherently reductionist, often representing only a subset of an organ’s or organism’s complexity, and thus potentially omitting systemic features present in whole animals. Additionally, the field lacks “gold standard” assays and validated protocols, making regulatory acceptance a work-in-progress.
Despite these hurdles, recent technological advances in 3D culture systems are rapidly improving the physiological relevance, reproducibility, and scalability of organoid-based assays. One such technology is the clinostat-based bioreactor, exemplified by CelVivo’s ClinoStar and ClinoReactor systems. These devices leverage clinostat principles – slow rotation to neutralize gravitational sedimentation – to cultivate 3D organoids in a suspension culture. With the new pivot, the need for human-relevant 3D models is only going to increase, providing big opportunities and requiring dedicated focus.
3D In Vitro Can Increase Efficiency and Efficacy
Transitioning away from animal models requires in vitro systems that replicate key aspects of human biology. Traditional two-dimensional (2D) cell cultures fall short in this regard – cells grown on flat plastic surface are stressed and lack the three-dimensional interactions present in real tissues. This can lead to misleading results; for example, drug responses in 2D cultures often poorly predict outcomes in animals or patients.
Within 3D culture methods, two primary approaches are generally used: static and dynamic systems. Static cultures, such as those embedded in hydrogels or grown in hanging droplets, often suffer from diffusion limitations that can cause nutrient deprivation and waste accumulation, leading to unphysiological hypoxia or necrosis in larger organoids. In contrast, dynamic systems like spinning flasks or orbital shakers improve nutrient delivery but typically introduce high shear forces, which can stress or damage the delicate cell structures.
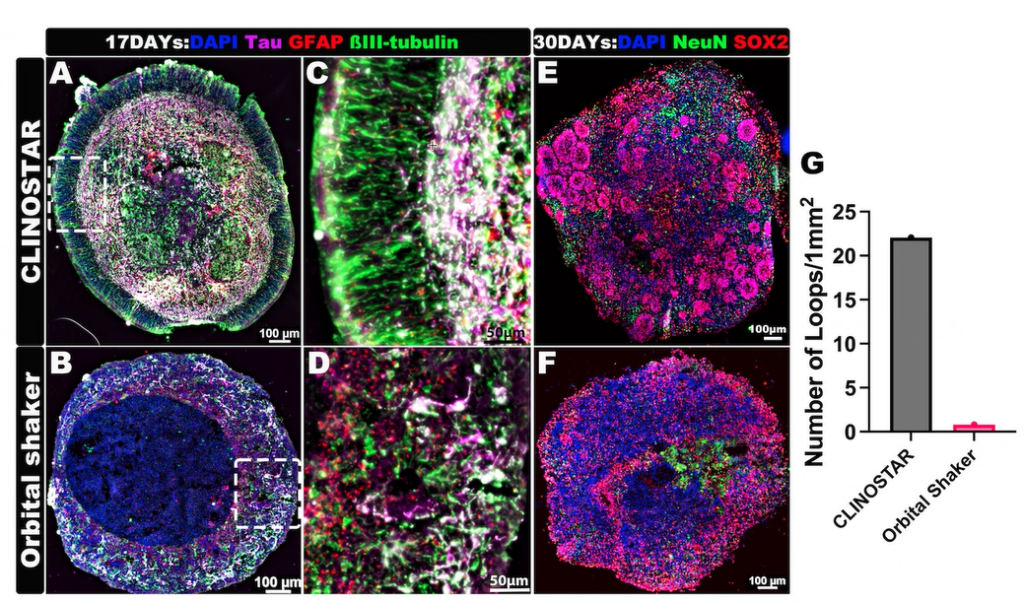
Brain organoids developing superior organization and functionality when cultured in a ClinoStar compared to an orbital shaker. Images by Liang Oscar Qiang, MD, PhD, Assistant Professor, Drexel University College of Medicine.
By contrast, 3D-clinostat organoid cultures allow cells to interact in all dimensions under low-shear, stress-free conditions, fostering more native-like cell polarity, functionality, and cell–cell communication. Organoids cultivated under clinostat conditions naturally develop oxygen, nutrient, and metabolite gradients, including hypoxic cores, similar to those in tumours and intact organs. These features make clinostat-grown organoids significantly more physiologically relevant than flat monolayers or organoids produced by traditional static or dynamic 3D methods. Indeed, growing cells in 3D-clinostat environments can induce functionality otherwise seen only at the whole-organ level – a paradigm shift in cell biology.
Need for Holistic Integration
Despite their promise, organoids present practical challenges that must be addressed to confidently replace animal studies. These cultures can be variable – different labs use different protocols, cell sources, and matrix strategies – leading to results that are difficult to compare between labs. Furthermore, because organoids recapitulate only a fraction of an organ’s function, they lack the holistic integration of an entire organism (e.g., neuro-immune or endocrine interactions).
These concerns are actively being tackled through community efforts to standardize 3D culture methods and to develop batteries of organoid-based representations of various organ systems and compatible assays. New computational tools and AI are also being explored to integrate data from multiple organoid models, although large reference datasets are still needed for model training.
Reproducibility: Key to Fulfilling Significant Impact
In this context, improving the reproducibility of organoid culture is critical. If hundreds of uniform organoids can be grown easily and yield consistent readouts, it becomes feasible to use them in screening assays and regulatory testing, much like animal models are used today. This is where clinostat-based bioreactors are making a significant impact.
Studies have demonstrated that organoids cultured under clinostat conditions exhibit reduced variability in size and cellular composition compared to traditional static or high-flow 3D methods (Stransky et al., 2022; Budeus et al., 2024). For example, Stransky and colleagues showed that spheroids generated using clinostat-based systems exhibited size coefficients of variation below 10%, enabling consistent responses to drug exposure across replicates. Similarly, Budeus et al. reported that clinostat-grown lung organoids demonstrated uniform structural and cellular features over extended culture periods, providing robust platforms for radiation toxicity testing.
Such consistency is pivotal for assay development and for regulatory validation of organoid models. We are already seeing clinostat-grown organoids being used to model diseases (from cancer to neurodegeneration) and to evaluate drug safety and efficacy in ways that were not feasible just a few years ago. As confidence in these systems grows, regulatory agencies may increasingly accept organoid-based data in lieu of animal studies, especially for modalities like monoclonal antibodies that have species-specific effects.
Conclusion and Outlook
Clinostat-based organoid culture systems such as CelVivo’s ClinoStar are accelerating the shift toward more human-relevant biomedical research. By enabling the growth of organ-mimetic, multi-cell type 3D tissues in vitro – under controlled and reproducible conditions – these technologies directly address key mandates of the FDA and scientific community: to refine research methods, reduce reliance on animal models, and ultimately replace animal testing where possible.
The practical benefits of clinostat 3D systems (high reproducibility, long-term viability, and easy integration with existing protocols) have lowered barriers for adoption, allowing many labs to enter the organoid field and generate human-relevant data more directly than conventional models.
There remain challenges to surmount – ensuring that organoid models are validated, establishing reference standards, and continuing to improve model complexity (for example, integrating immune components or vasculature into organoids). Yet, the progress to date has been encouraging. Each technical improvement and each successful application in translational research represent a step up the proverbial “mountain range” towards fully animal-free biomedical science. The endorsement of alternative methods by the FDA and NIH has catalyzed this journey, and technologies like the ClinoStar are proving to be invaluable tools for navigating it.
With a slightly advocacy-oriented view, one can argue that investing in and embracing 3D clinostat culture now will pay off in more predictive, relevant, and innovative research outcomes – bringing us closer to the day when lab-grown mini-organs, not animal models, are the gold standard for drug development and disease modelling.
